Stereoselective synthesis of protectin D1: a potent anti-inflammatory and proresolving lipid mediator†
Abstract
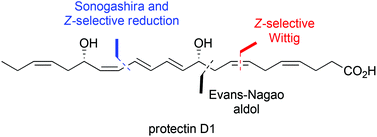
A convergent stereoselective synthesis of the potent anti-inflammatory, proresolving and neuroprotective lipid mediator protectin D1 (2) has been achieved in 15% yield over eight steps. The key features were a stereocontrolled Evans-aldol reaction with Nagao's chiral auxiliary and a highly selective Lindlar reduction of internal alkyne 23, allowing the sensitive conjugated E,E,Z-triene to be introduced late in the preparation of 2. The UV and LC/MS–MS data of synthetic protectin D1 (2) matched those obtained from endogenously produced material.

 Please wait while we load your content...
Please wait while we load your content...