Metal free stereoselective synthesis of functionalized enamides†
Abstract
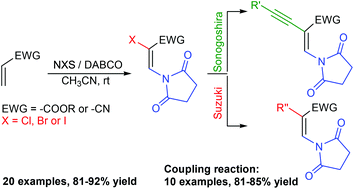
An efficient and expeditious DABCO-mediated synthesis of functionalized enamides from alkenes is delineated. The reaction proceeds through an unprecedented cascade involving an Aza-Michael addition/α-bromination/elimination and a Morita–Baylis–Hillman type reaction to generate functionalized enamides in a regio- & stereoselective fashion.

 Please wait while we load your content...
Please wait while we load your content...