Dimerization of a heat shock protein 90 inhibitor enhances inhibitory activity†
Abstract
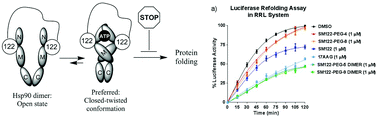
Heat shock protein 90 (hsp90) accounts for 1–2% of the total proteins in normal cells and it functions as a dimer. Hsp90 behaves as a molecular chaperone that folds, assembles, and stabilizes client proteins. We have developed a novel hsp90 inhibitor, and herein we describe the synthesis and biological activity of the dimerized variant of this inhibitor. Tethering a monomer inhibitor together produced a dimerized compound that more effectively inhibits hsp90 over the monomer.

 Please wait while we load your content...
Please wait while we load your content...