Fast-pulsing NMR techniques for the detection of weak interactions: successful natural abundance probe of hydrogen bonds in peptides†
Abstract
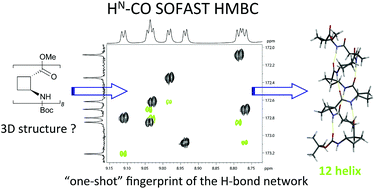
Structural investigations of peptides using NMR spectroscopy rarely include the detection of N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H⋯N hydrogen bonds, because the relevant heteronuclei have a low natural abundance while the small trans hydrogen bond scalar couplings reduce the sensitivity. Fast repetition NMR techniques combined with state of the art spectrometer specifications allowed the enhancement of the sensitivity for detection of hydrogen bonds at natural isotopic abundance.
C and N–H⋯N hydrogen bonds, because the relevant heteronuclei have a low natural abundance while the small trans hydrogen bond scalar couplings reduce the sensitivity. Fast repetition NMR techniques combined with state of the art spectrometer specifications allowed the enhancement of the sensitivity for detection of hydrogen bonds at natural isotopic abundance.

 Please wait while we load your content...
Please wait while we load your content...