Structure–immunogenicity relationship of kresoxim-methyl regioisomeric haptens†
Abstract
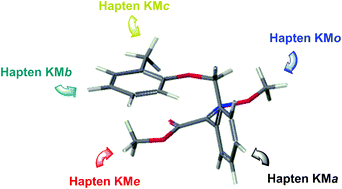
Kresoxim-methyl was one of the two first strobilurins to be discovered, and nowadays it is widely used as an antifungal agent in crop protection. Because of its low molecular weight and negligible structural complexity, the generation of antibodies to kresoxim-methyl noticeably requires the preparation of functionalized haptens. In this study, the introduction of a hydrocarbon spacer arm at the aromatic moieties of the target molecule was carried out by a convergent strategy based on the Sonogashira cross-coupling reaction, and functionalized linkers of the same length were also tethered to the aliphatic toxophore group by the O-alkylation reaction. Evaluation of the immune response, in terms of antibody affinity, showed a differential behavior among five synthesized haptens whose sole dissimilarity was the derivatization site. The characteristic (methoxyimino)acetate moiety of strobilurins was revealed as the optimum linker position for high-affinity polyclonal and monoclonal antibody production. However, good monoclonal antibodies were isolated from mice immunized with a hapten carrying the linker at an opposite site, which otherwise generated a poor polyclonal response in rabbits. Site-heterology was confirmed as a feasible approach for the improvement of the apparent affinity, particularly with polyclonal antibodies. Several of the monoclonal antibodies generated in the context of this project could be proper binders for kresoxim-methyl immunosensing over different analytical platforms.

 Please wait while we load your content...
Please wait while we load your content...