Stereoselective preparation of lipidated carboxymethyl-proline/pipecolic acid derivatives via coupling of engineered crotonases with an alkylmalonyl-CoA synthetase†
Abstract
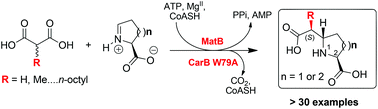
The trisubstituted enolate- and C–C bond-forming capacities of engineered carboxymethylproline synthases CMPSs are coupled with the malonyl-CoA synthetase MatB to enable stereoselective preparation of 5- and 6-membered N-heterocycles functionalised with alkyl-substituted carboxymethyl side chains, starting from achiral alkyl-substituted malonic acids and L-amino acid semialdehydes. The results illustrate the biocatalytic utility of crotonases in tandem enzyme-catalysed reactions for stereoselective synthesis.

 Please wait while we load your content...
Please wait while we load your content...