Histidine-functionalized water-soluble nanoparticles for biomimetic nucleophilic/general-base catalysis under acidic conditions†
Abstract
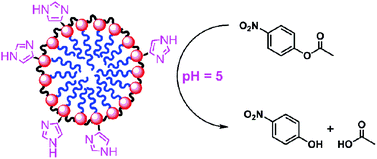
Cross-linking the micelles of 4-dodecyloxybenzyltripropargylammonium bromide by 1,4-diazidobutane-2,3-diol in the presence of azide-functionalized imidazole derivatives yielded surface-cross-linked micelles (SCMs) with imidazole groups on the surface. The resulting water-soluble nanoparticles were found, by fluorescence spectroscopy, to contain hydrophobic binding sites. The imidazole groups promoted the photo-deprotonation of 2-naphthol at pH 6 and catalyzed the hydrolysis of p-nitrophenylacetate (PNPA) in aqueous solution at pH ≥ 4. Although the overall hydrolysis rate slowed down with decreasing solution pH, the catalytic effect of the imidazole became stronger because the reactions catalyzed by unfunctionalized SCMs slowed down much more. The unusual ability of the imidazole–SCMs to catalyze the hydrolysis of PNPA under acidic conditions was attributed to the local hydrophobicity and the positive nature of the SCMs.

 Please wait while we load your content...
Please wait while we load your content...