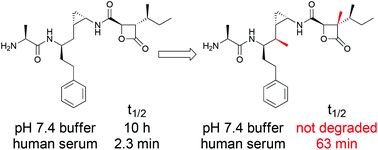
The belactosin A analog 2a, having the unnatural cis-cyclopropane structure instead of the trans-cyclopropane structure in belactosin A, is a much more potent proteasome inhibitor than belactosin A. However, its cell growth inhibitory effect is rather lower than that expected from its remarkable proteasome inhibitory effect, probably due to its instability under cellular conditions. We hypothesized that the instability of 2a was due to chemical and enzymatic hydrolysis of the strained β-lactone moiety. Thus, to increase the stability of 2a by chemical modification, its analogs with a sterically more hindered β-lactone moiety and/or cyclopropylic strain-based conformational restriction were designed and synthesized, resulting in the identification of a stabilized analog 6a as a proteasome inhibitor with cell growth inhibitory effects. Our findings suggest that the chemical and biological stability of 2a is significantly affected by the steric hindrance around its β-lactone carbonyl moiety and the conformational flexibility of the molecule.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...