Copper(ii) chloride mediated (aza)oxindole synthesis by oxidative coupling of Csp2–H and Csp3–H centers: substrate scope and DFT study†
Abstract
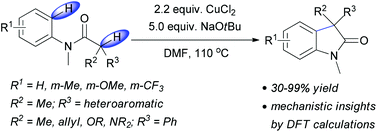
A CuCl2 mediated direct intramolecular oxidative coupling of Csp2–H and Csp3–H centers gives access to 3,3-disubstituted

 Please wait while we load your content...
Please wait while we load your content...