Synthesis of (2-alkylthiothiazolin-5-yl)methyl dodecanoates via tandem radical reaction†
Abstract
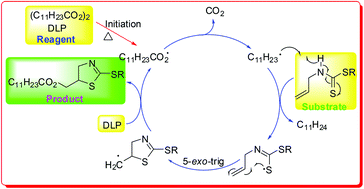
A series of (2-alkylthiothiazolin-5-yl)methyl dodecanoates was synthesized from various alkyl N-allylcarbamodithioates and dilauroyl peroxide via a tandem radical hydrogen-abstraction–cyclization–substitution/combination reaction with a 5-exo-trig radical cyclization as a key step. The current route is the first, convenient, and efficient synthesis of (2-alkylthiothiazolin-5-yl)methanol derivatives.

 Please wait while we load your content...
Please wait while we load your content...