Stereoselective synthesis of Arabidopsis CLAVATA3 (CLV3) glycopeptide, unique protein post-translational modifications of secreted peptide hormone in plant†
Abstract
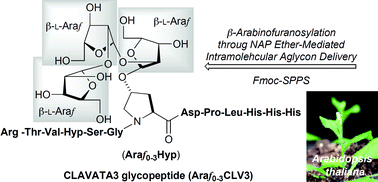
The unique hydroxylproline (Hyp)-linked O-glycan modification is a common process in hydroxyproline-rich glycoproteins (HRGPs). The modification occurs through post-translational hydroxylation at 4-position of proline residues some of which are followed by O-glycosylation at the resulting Hyp which is also found in some secreted peptide hormones such as CLAVATA3 (CLV3) of Arabidopsis thaliana plants. An active mature CLV3 is a tridecapeptide linked to β-L-Araf-(1→2)-β-L-Araf-(1→2)-β-L-Araf at a Hyp residue in the center of the peptide sequence such as Arg-Thr-Val-Hyp-Ser-Gly-Hyp(L-Arafn)-Asp-Pro-Leu-His-His-His (n = 3). We report here the synthesis of the secreted and modified CLV3 glycopeptide with all glycoforms (Araf0–3CLV3) of A. thaliana plants. A highly stereoselective β-arabinofuranosylation of Hyp derivatives as the key step of the synthesis of CLV3 glycopeptide was achieved by NAP ether-mediated IAD, which was effectively applied to the synthesis of oligoarabinosylated hydroxylproline [Hyp(L-Araf1–3)] derivatives. Fmoc-solid phase peptide synthesis was carried out using COMU as the coupling reagent for the introduction of [Hyp(L-Araf0–3)] derivatives as well as further elongation to the CLV3 glycopeptides.

 Please wait while we load your content...
Please wait while we load your content...