Reaction pathways and free energy profiles for spontaneous hydrolysis of urea and tetramethylurea: unexpected substituent effects†
Abstract
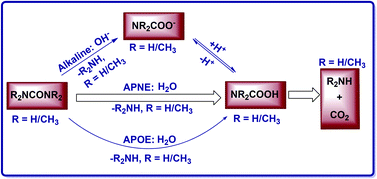
It has been difficult to directly measure the spontaneous hydrolysis rate of urea and, thus, 1,1,3,3-tetramethylurea (Me4U) was used as a model to determine the “experimental” rate constant for urea hydrolysis. The use of Me4U was based on an assumption that the rate of urea hydrolysis should be 2.8 times that of Me4U hydrolysis because the rate of acetamide hydrolysis is 2.8 times that of N,N-dimethyl-acetamide hydrolysis. The present first-principles electronic-structure calculations on the competing non-enzymatic hydrolysis pathways have demonstrated that the dominant pathway is the neutral hydrolysis via the CN addition for both urea (when pH < ∼11.6) and Me4U (regardless of pH), unlike the non-enzymatic hydrolysis of amides where alkaline hydrolysis is dominant. Based on the computational data, the substituent shift of the free energy barrier calculated for the neutral hydrolysis is remarkably different from that for the alkaline hydrolysis, and the rate constant for the urea hydrolysis should be ∼1.3 × 109-fold lower than that (4.2 × 10−12 s−1) measured for the Me4U hydrolysis. As a result, the rate enhancement and catalytic proficiency of urease should be 1.2 × 1025 and 3 × 1027 M−1, respectively, suggesting that urease surpasses proteases and all other enzymes in its power to enhance the rate of reaction. All of the computational results are consistent with available experimental data for Me4U, suggesting that the computational prediction for urea is reliable.

 Please wait while we load your content...
Please wait while we load your content...