An unusual 1,2-aryl shift in palladium-catalyzed cross-coupling ethoxycarbonylation of arylboronic acids with α-iminoesters†
Abstract
The Pd-catalyzed cross-coupling ethoxycarbonylation of aryl boronic acids with N-aryl-α-iminoesters affords aryl carboxylic esters via carbonyl-imino σ bond cleavage. This unprecedented mode of reaction allows regioselective installation of the
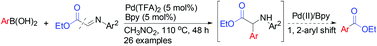

 Please wait while we load your content...
Please wait while we load your content...