Synthesis of azetidines and pyrrolidinesvia iodocyclisation of homoallyl amines and exploration of activity in a zebrafish embryo assay†
Abstract
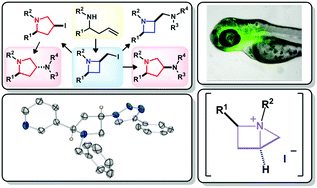
Room temperature iodocyclisation of homoallylamines stereoselectively delivers functionalised 2-(iodomethyl)azetidine derivatives in high yield. Increasing reaction temperature from 20 °C to 50 °C switches the reaction outcome to realise the stereoselective formation of functionalised 3-iodopyrrolidine derivatives. It was shown that these

 Please wait while we load your content...
Please wait while we load your content...