Tuning activity-based probe selectivity for serine proteases by on-resin ‘click’ construction of peptidediphenyl phosphonates†
Abstract
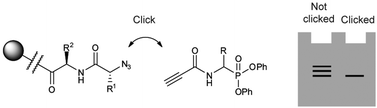
Activity-based probes (ABPs) are powerful tools for functional proteomics studies. Their selectivity can be influenced by modification of a recognition element that interacts with pockets near the active site. For

 Please wait while we load your content...
Please wait while we load your content...