Formation of tetrahydrofurans via a 5-endo-tet cyclization of aziridines – synthesis of (−)-pachastrissamine†
Abstract
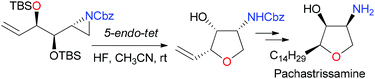
The formation of tetrahydrofurans from 2-hydroxyalkyl-oxirane or aziridine is reported. The 5-endo-tet cyclization/ring opening of aziridine proceeded smoothly to give tetrahydrofurans (THFs) under mild conditions. In contrast, the corresponding process of oxirane was unsuccessful and a sequence of SN2 substitution/cyclization was required to form THFs. The application of the process to prepare ent-(−)-pachastrissamine is described.

 Please wait while we load your content...
Please wait while we load your content...