Convenient in situ generation of various dichlorinating agents from oxone and chloride: diastereoselective dichlorination of allylic and homoallylic alcohol derivatives†
Abstract
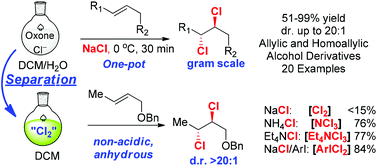
A safe and convenient protocol was developed for in situ generation of various dichlorinating agents (cf. Cl2, NCl3, Et4NCl3, ArICl2) from

 Please wait while we load your content...
Please wait while we load your content...