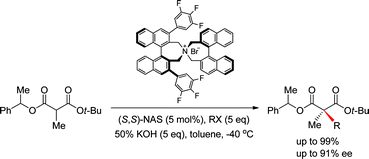
A new asymmetric synthetic method to prepare α,α-dialkylmalonates for the construction of a quaternary carbon center via phase-transfer catalytic (PTC) alkylation has been developed. Enantioselective α-alkylation of 2-methylbenzyl tert-butyl α-methylmalonates under phase-transfer catalytic conditions in the presence of (S,S)-3,4,5-trifluorophenyl-NAS bromide (10) afforded the corresponding α,α-dialkylmalonates in high chemical (up to 99%) and optical yields (up to 91% ee), which were selectively hydrolyzed to malonic monoacids under alkali basic conditions for conversion to versatile chiral intermediates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...