Scope and limitations of the Heck–Matsuda-coupling of phenol diazonium salts and styrenes: a protecting-group economic synthesis of phenolic stilbenes†
Abstract
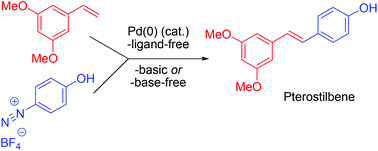
4-Phenol diazonium salts undergo Pd-catalyzed Heck reactions with various styrenes to 4′-hydroxy stilbenes. In almost all cases higher yields and fewer side products were observed, compared to the analogous 4-methoxy benzene diazonium salts. In contrast, the reaction fails completely with 2- and 3-phenol diazonium salts. For these substitution patterns the methoxy-substituted derivatives are superior.

 Please wait while we load your content...
Please wait while we load your content...