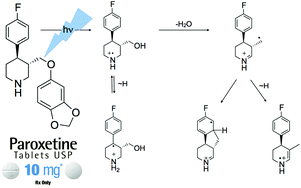
Quantum chemical calculations have been used to model reactions which are important for understanding the chemical fate of paroxetine-derived radicals in the environment. In order to explain the experimental observation that the loss of water occurs along the (photo)degradation pathway, four different mechanisms of radical-induced dehydrations have been considered. The elimination of water from the N-centered radical cation, which results in the formation of an imine intermediate, has been calculated as the most feasible process. The predicted energy barrier (ΔG#298 = 98.5 kJ mol−1) is within the barrier limits set by experimental measurements. All reaction intermediates and transition state structures have been calculated using the G3(MP2)-RAD composite procedure, and solvent effects have been determined using a mixed (cluster/continuum) solvation model. Several new products, which comply with the available experimental data, have been proposed. These structures could be relevant for the chemical fate of antidepressant paroxetine, but also for biologically and environmentally related substrates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...