A stereoselective synthesis of the C9–C19 subunit of (+)-peloruside A†
Abstract
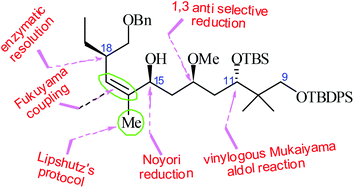
The stereoselective synthesis of a C9–C19 fragment of the potent antitumor agent peloruside A is disclosed. The C11 stereogenic centre was created by a vinylogous Mukaiyama aldol reaction following Carreira's protocol, with excellent stereocontrol. The C13 stereogenic centre was introduced by a substrate controlled

 Please wait while we load your content...
Please wait while we load your content...