Synthesis of 2,4-unsubstituted quinoline-3-carboxylic acid ethyl esters from arylmethyl azides via a domino process†
Abstract
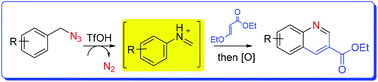
A convenient synthesis of 2,4-unsubstituted quinoline-3-carboxylic acid ethyl esters via a domino process is described. The synthesis employs arylmethyl azides as the precursor which undergoes an acid-promoted rearrangement to give an N-aryl iminium ion. Following the addition with

 Please wait while we load your content...
Please wait while we load your content...