Total synthesis of (±)-sacidumlignans D and A through Ueno–Stork radical cyclization reaction†
Abstract
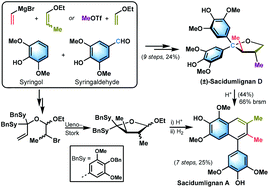
Efficient synthesis of (±)-sacidumlignan D (4) has been successfully achieved employing Ueno–Stork radical cyclization of α-bromo acetal 21 as a key step. Two synthetic approaches for the symmetrical diaryl

 Please wait while we load your content...
Please wait while we load your content...