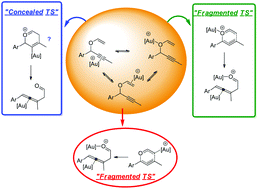
Computational and experimental analysis of unusual substituent effects in the Au-catalyzed propargyl Claisen rearrangement revealed new features important for the future development of Au(I) catalysis. Despite the higher stability of Au–alkyne complexes, they do not always correspond to the catalytically active compounds. Instead, the product emanates from the higher energy Au(I)–oxygen complex reacting via a low barrier cation-accelerated oxonia Claisen pathway. Additionally, both intra and intermolecular competition from other Lewis bases present in the system, for the Au(I) catalyst, can lead to unproductive stabilization of the substrate/catalyst complex, explaining hitherto unresolved substituent effects.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...