A rotaxane host system containing integrated triazole C–H hydrogen bond donors for anion recognition†
Abstract
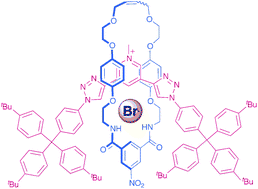
Two rotaxanes incorporating a 3,5-bis(triazole)-pyridinium axle component have been prepared using either an anion templated amide condensation or ring closing metathesis (RCM) clipping strategy. The respective yields of interlocked receptor were found to be significantly higher when the RCM clipping synthetic route was used. Proton NMR titration experiments in competitive 1 : 1 CDCl3–CD3OD solvent media reveal that the rotaxane prepared by the clipping procedure is selective for halide anions over larger, more basic oxoanions. Interestingly, the interlocked host displays an unusual preference for bromide over other halide anions.
- This article is part of the themed collections: In celebration of Paul Beer’s 60th Birthday and In Celebration of Andrew D. Hamilton’s Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...