Complementary regioselectivity in the synthesis of iminohydantoins: remarkable effect of amide substitution on the cyclization†‡
Abstract
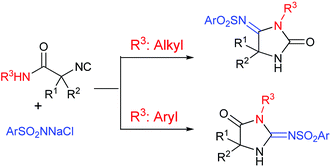
Complementary regioselective synthesis of iminohydantoins from isocyanoacetamides controlled by the substituent on the amide group has been described. 4-Iminohydantoins were the major products when the starting materials were N-alkyl isocyanoamides, whereas 2-iminohydantoins were the major products with N-aryl isocyanoamides.

 Please wait while we load your content...
Please wait while we load your content...