3,5-Bis(acetaldehyde) substituted BODIPY†
Abstract
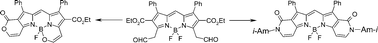
Two versions of a facile and efficient synthetic approach to 3,5-bis(acetaldehyde) substituted BODIPY have been developed and this compound has been used to obtain, in high yields, a variety of 3,5-divinyl BODIPY derivatives (vinylacetates, enamines, vinyl chlorides, and some multiple-functionalized products). As shown, vinyl substituents have an additive effect on the position of the BODIPY absorption maximum. The dyes synthesized are quite stable and exhibit intense absorption and fluorescence with wavelengths longer than 600 nm.

 Please wait while we load your content...
Please wait while we load your content...