Nucleophilic substitution of bromonorbornenes and derivatives by electron transfer reactions†
Abstract
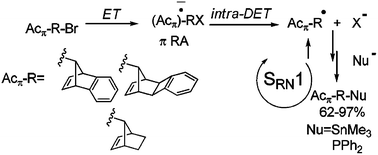
The photoinitiated substitution reactions of anti-7-bromobenzonorbornadiene (5), its syn isomer 6, exo–anti-13-bromobenzocyclobutanorbornene (7), syn-7-bromonorbornene (8) and bromonorbornane (9) with Me3Sn− and Ph2P− anions, in liquid ammonia, are here informed to occur with good yields of substitution. The stereochemical outcome is discussed in terms of calculations with the B3LYP functional and the 6-31+G* basis set; the solvent being included as a continuum through the PCM model. The experimental relative chemical reactivity of pairs of substrates toward a given anion is also presented.

 Please wait while we load your content...
Please wait while we load your content...