A further study of acetylacetone nitrosation†
Abstract
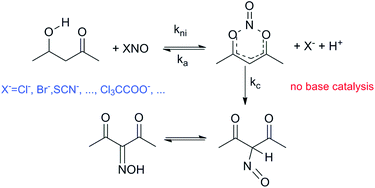
The nitrosation of acetylacetone (AcAc) has been revised in an aqueous acid medium of perchloric acid and buffers of mono-, di-, or tri-chloroacetic acid. The results show that in the presence of buffers, under conditions of [nit] ≪ [AcAc] (nit = sodium nitrite) the reaction cannot be studied by UV-Vis spectroscopy, contrary to the recently published paper by García-Rio et al. (J. Org. Chem., 2008, 73, 8198). The present study also corroborates the previously published mechanism of AcAc nitrosation, where no base-catalysis was observed. Contrarily, the low effect of buffers was attributed to the formation of nitrosyl chloro-, dichloro- or trichloro-acetate salts that are new nitrosating agents.

 Please wait while we load your content...
Please wait while we load your content...