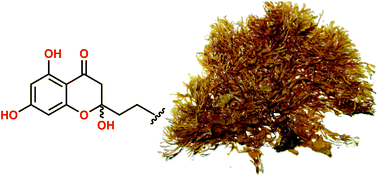
An intertidal sample of the Australian marine brown alga, Zonaria spiralis, exhibited promising kinase inhibitory and antibacterial activity. Chemical analysis returned six phloroglucinol-derived lipids, the new hemiketal spiralisones A–D (1–4) and the known chromones 5–6, and the known norsesquiterpenoid apo-9′-fucoxanthinone (7). Structures 1–7 were assigned on the basis of detailed spectroscopic analysis, biosynthetic considerations and total synthesis. Spiralisones undergo facile acid-mediated dehydration to yield the corresponding chromones, revealing for the first time that brown algal chromones may be handling artifacts rather than natural products. Hemiketals 1 and 2, and chromone 6, displayed inhibitory activity against the neurodegenerative disease kinase targets CDK5/p25, CK1δ and GSK3β, while hemiketals 1, 3 and 4, and chromone 6, displayed growth inhibitory activity against the Gram-positive bacteria Bacillus subtilis (ATCC 6051 and 6633). The promising kinase inhibitory and antibacterial properties of the Z. spiralis extract were attributed to the cumulative effect of many moderately potent phloroglucinol-derived lipid co-metabolites.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...