The stereochemical outcome of allyl magnesium and indium additions to 5-substituted norbornen-7-ones and its application to cis fused carbocycle formation viaring rearrangement metathesis†
Abstract
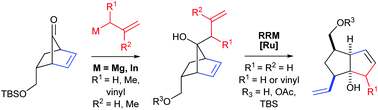
The addition of allyl magnesium and allyl indium reagents to a key TBS protected norbornenyl building block, synthesised in 6-steps from commercially available

 Please wait while we load your content...
Please wait while we load your content...