Self-assembling properties of all γ-cyclic peptides containing sugar amino acid residues†
Abstract
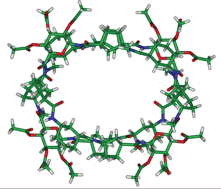
In this study, a novel dimer-forming cyclic peptide composed exclusively by cyclic γ-amino acids with a saccharide-like outer surface is described. The antiparallel β-sheet type hydrogen bonding interactions responsible for the large association constant in non-polar solvents constitute a suitable model for a novel class of self-assembling peptide nanotubes.

 Please wait while we load your content...
Please wait while we load your content...