Solid phase synthesis of peptides containing backbone-fluorinated amino acids†
Abstract
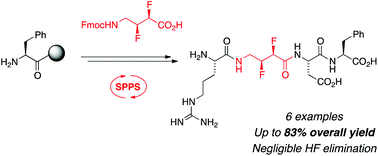
Backbone-fluorinated amino acids exhibit unique conformational behaviour, and have potential utility as components of bioactive shape-controlled peptides. However, methods for the elaboration of backbone-fluorinated amino acids have thus far been limited to solution phase peptide coupling reactions. In this paper, protocols are developed that allow the successful manipulation of backbone-fluorinated amino acids using Fmoc-strategy solid phase peptide synthesis. To exemplify this strategy, several fluorinated RGD peptide analogues were synthesised in moderate to good overall yields.

 Please wait while we load your content...
Please wait while we load your content...