Synthesis of novel diosgenyl saponin analogues and apoptosis-inducing activity on A549 human lung adenocarcinoma†
Abstract
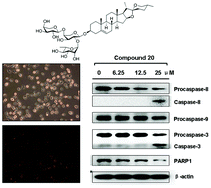
We synthesized a diosgenyl saponin bearing a unique disaccharide from the natural product β-hederin, together with twelve glycosylated derivatives and determined their cytotoxicity against five different human cancer cell lines. Most of them showed weak cytotoxicity, with the exception of compound 20, diosgenyl α-L-rhamnopyranosyl-(1→2)-[α-L-arabinopyranosyl-(1→4)]-α-L-arabinopyranoside, which exhibited strong cytotoxicity against A549 cells. The cytotoxicity of 20 was associated with apoptotic cell death, which was characterized by morphological changes, chromatin condensation, DNA fragmentation, and phosphatidylserine externalization. Compound 20 induced apoptosis of A549 cells through a caspase-8-mediated extrinsic pathway and a caspase-9-mediated intrinsic pathway. In addition, phosphorylation of JNK increased but the phosphorylation of ERK decreased after treatment with 20. These results provide a basic mechanism for the anticancer activity of 20.

 Please wait while we load your content...
Please wait while we load your content...