On the mechanism of the Dakin–West reaction†
Abstract
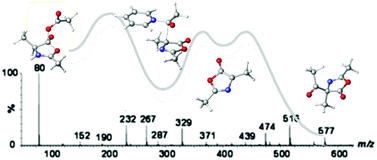
The mechanism of the Dakin–West reaction has been thoroughly investigated by monitoring the reaction using ESI-MS/MS techniques in combination with M06-2X/6-311++G(d,p) calculations. Several of the key intermediates in the previously proposed “azlactone” mechanism have been experimentally detected and characterized. In particular, interception of the mixed anhydrides involved in the early and late stages of the mechanistic scheme, as well as of the cyclic acyl-oxazolone intermediate, supports the original pathway suggested by Dakin and West. All intermediates and transition structures involved in several competing mechanisms have been calculated. The theoretical calculations support the experimental results and corroborate the proposed “azlactone” mechanism. The pathway involving the cyclic oxazolone (“azlactone”) intermediate represents an energy barrier more than 3 kcal mol−1 lower than for the competing aldol-type mechanism, thus ruling out this alternative mechanism. The DFT calculations explain the observed ESI-MS data and assess those intermediates which the experiments cannot fully elucidate.

 Please wait while we load your content...
Please wait while we load your content...