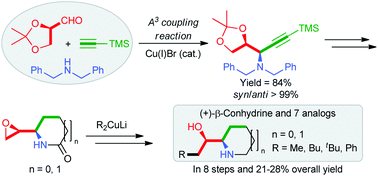
Diastereoselective construction of syn-α-oxyamines via three-component α-oxyaldehyde–dibenzylamine–alkyne coupling reaction: application in the synthesis of (+)-β-conhydrine and its analogues†
Abstract
A Cu(I)-catalyzed α-oxyaldehyde–dibenzylamine–alkyne

 Please wait while we load your content...
Please wait while we load your content...