Camphor-derived C1-symmetric chiral diamine organocatalysts for asymmetric Michael addition of nitroalkanes to enones†
Abstract
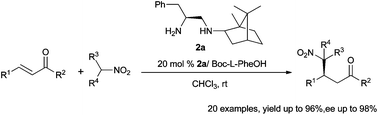
A series of stable C1-symmetric chiral diamines (2a–2l) were conveniently synthesized by condensing exo-(−)-bornylamine or (+)-(1S,2S,5R)-menthylamine with various commercially available Cbz-protected amino acids. Among them, 2a can efficiently promote the Michael addition of nitroalkanes to a broad scope of enones with high yields (up to 96%) and excellent enantioselectivities (up to 98%) under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...