The origin of global and macrocyclic aromaticity in porphyrinoids
Abstract
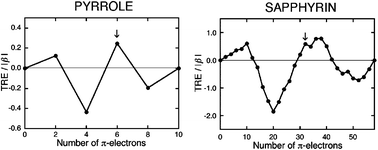
The global and macrocyclic aromaticity of porphyrinoids was characterized using our graph theory of aromaticity. The sequential line plots of topological resonance energy (TRE) against the number of π-electrons (Nπ) for different porphyrinoids are similar with four major extrema to those for five-membered heterocycles. This supports the view that five-membered rings are the main origin of global aromaticity in porphyrinoids. Macrocyclic circuits contribute significantly to macrocyclic π-circulation but modestly to global aromaticity. Macrocyclic aromaticity/antiaromaticity in oligopyrrolic

 Please wait while we load your content...
Please wait while we load your content...