Peripherally ethynylated carbazole-based core-modified porphyrins†
Abstract
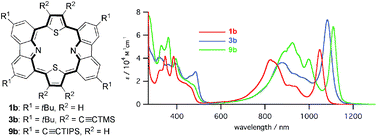
Peripherally ethynylated carbazole-based core-modified porphyrins were synthesized by sequential metal-catalyzed coupling and annulation reactions. Experimental results and DFT calculations both confirm that the π-conjugated networks of the resulting porphyrins effectively delocalize over the entire macrocycle, including the ethynyl substituent groups.

 Please wait while we load your content...
Please wait while we load your content...