Efficient proline and prolinol ether mediated 3-component synthesis of 3- and 3,4-substituted chromenone derivatives†
Abstract
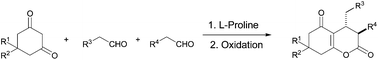
A highly efficient route for the synthesis of valuable 3,4-substituted
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...