An enzyme-initiated Smiles rearrangement enables the development of an assay of MshB, the GlcNAc-Ins deacetylase of mycothiol biosynthesis†
Abstract
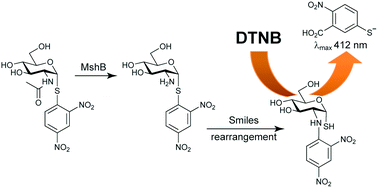
MshB is the N-acetyl-1-D-myo-inosityl-2-amino-2-deoxy-D-glucopyranoside (GlcNAc-Ins) deacetylase active as one of the enzymes involved in the biosynthesis of mycothiol (MSH), a protective low molecular weight thiol present only in Mycobacterium tuberculosis and other actinomycetes. In this study, structural analogues of GlcNAc-Ins in which the inosityl moiety is replaced by a chromophore were synthesized and evaluated as alternate substrates of MshB, with the goal of identifying a compound that would be useful in high-throughput assays of the enzyme. In an unexpected and surprising finding one of the GlcNAc-Ins analogues is shown to undergo a Smiles rearrangement upon MshB-mediated deacetylation, uncovering a free thiol group. We demonstrate that this chemistry can be exploited for the development of the first continuous assay of MshB activity based on the detection of thiol formation by DTNB (Ellman's reagent); such an assay should be ideally suited for the identification of MshB inhibitors by means of high-throughput screens in microplates.

 Please wait while we load your content...
Please wait while we load your content...