Synthesis of isoxazolesen route to semi-aromatized polyketides: dehydrogenation of benzonitrile oxide–para-quinone acetal cycloadducts†‡
Abstract
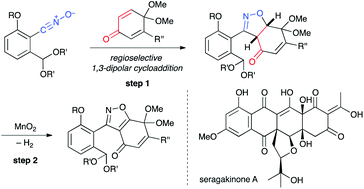
A variety of highly functionalized polycyclic
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...