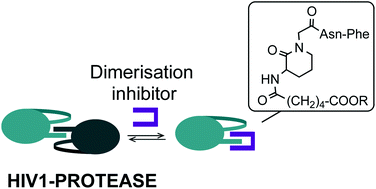
Applications of 3-aminolactams: design, synthesis, and biological evaluation of a library of potential dimerisation inhibitors of HIV1-protease†
Abstract
In the context of our studies on the applications of 3-aminolactams as conformationally restricted pseudodipeptides, we report here the synthesis of a library of potential dimerisation
 Please wait while we load your content...
Please wait while we load your content...