Synthesis and preliminary biological evaluation of carba analogues from Neisseria meningitidis A capsular polysaccharide†
Abstract
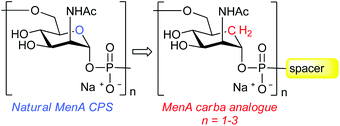
The Gram-negative encapsulated bacterium Neisseria meningitidis type A (MenA) is a major cause of meningitis in developing countries, especially in the sub-Saharan region of Africa. The development and manufacture of an efficient glycoconjugate vaccine against MenA is greatly hampered by the poor hydrolytic stability of its capsular polysaccharide, consisting of (1→6)-linked 2-acetamido-2-deoxy-α-D-mannopyranosyl phosphate repeating units. The replacement of the ring oxygen with a methylene group to get a carbocyclic analogue leads to the loss of the acetalic character of the phosphodiester and consequently to the enhancement of its chemical stability. Here we report the synthesis of oligomers (mono-, di- and trisaccharide) of carba-N-acetylmannosamine-1-O-phosphate as candidates for stabilized analogues of the corresponding fragments of MenA capsular polysaccharide. Each of the synthesized compounds contains a phosphodiester-linked aminopropyl spacer at its reducing end to allow for protein conjugation. The inhibition abilities of the synthetic molecules were investigated by a competitive ELISA assay, showing that only the carba-disaccharide is recognized by a polyclonal anti-MenA serum with an affinity similar to a native MenA oligosaccharide with average polymerization degree of 3.

 Please wait while we load your content...
Please wait while we load your content...