Folding-promoted TBAX-mediated selective demethylation of methoxybenzene-based macrocyclic aromatic pentamers
Abstract
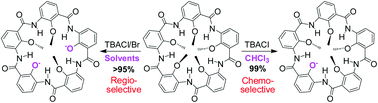
Described in this study is the ability of tetrabutylammonium salts (TBAX) to mediate an efficient mono- or di-demethylation removing one or two out of five aromatic methoxy methyl groups situated in similar chemical microenvironments in a H-bonded macrocyclic aromatic pentamer. These demethylations are found to be both chemo- and regioselective, and promoted by the H-bonding directed folding of the macrocyclic backbone.
 Please wait while we load your content...
Please wait while we load your content...