Abstract
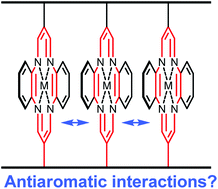
Three double stranded polymeric ladderphanes with 16-π-electron antiaromatic metallocycle linkers are synthesised by ring opening metathesis polymerisation of the corresponding bisnorbornene monomers. Scanning tunnelling microscopic (STM) images indicate that these polymers can assemble nicely on a graphite surface to form a highly ordered pattern which has been observed in other ladderphanes with different kinds of aromatic linkers. Little change in 1H NMR, absorption spectra and electrochemical oxidation potential between these polymers and the corresponding monomers suggest that there would be no interactions between adjacent antiaromatic linkers in these polymeric ladderphanes. Presumably, the distance between two antiaromatic rings in these ladderphanes (5–6 Å) is far too long in comparison with that between two rings in methylene-bridged antiaromatic superphanes (2.5 Å<), where stabilisation is predicted by theoretical calculations.
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...