New fluoride-promoted hypoiodite-catalytic oxidative cycloetherification to aromatic spiroketals†
Abstract
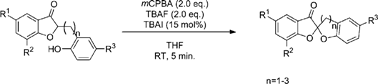
A new catalytic application of hypoiodite reagents generated in situ from iodide ions is found, which succeeded in the synthesis of bisbenzannelated

 Please wait while we load your content...
Please wait while we load your content...