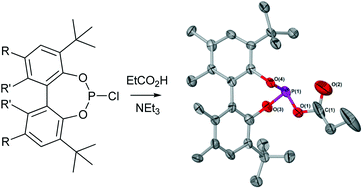
Simple mixed anhydrides are known to pose synthetic difficulties relating to their thermal lability and ways to stabilise such mixed anhydride systems by relying on either electronic or steric effects were therefore explored. Thus, a series of acyloxyphosphines and acylphosphites derived from either propanoic acid or phenylacetic acid were prepared and their in solution stability assessed. These compounds were, where stability allowed, fully characterised using standard analytical techniques. NMR studies, in particular, unearthed interesting coupling behaviour for a number of the acyloxyphosphines and acylphosphites as well as their rearrangement products which could be linked to their chiral nature. Furthermore, the crystal structures for three of the prepared mixed anhydrides were determined using X-ray crystallography and are reported herein.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...