Intramolecular reductive ketone–alkynoate coupling reaction promoted by (η2-propene)titanium†
Abstract
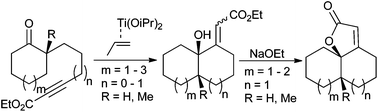
Intramolecular reductive coupling of cycloalkanones tethered to alkynoates in the presence of (η2-propene)titanium was successfully performed to provide hydroxy-esters in a diastereoselective manner. Subsequent lactonization afforded angularly fused unsaturated tricyclic

 Please wait while we load your content...
Please wait while we load your content...