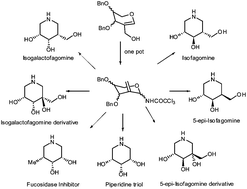
Aza-Claisen rearrangement of 2-C-hydroxymethyl glycals as a versatile strategy towards synthesis of isofagomine and related biologically important azasugars†
Abstract
Synthesis of isofagomine has been achieved by implementation of aza-Claisen rearrangement of 2-C-hydroxymethyl
 Please wait while we load your content...
Please wait while we load your content...